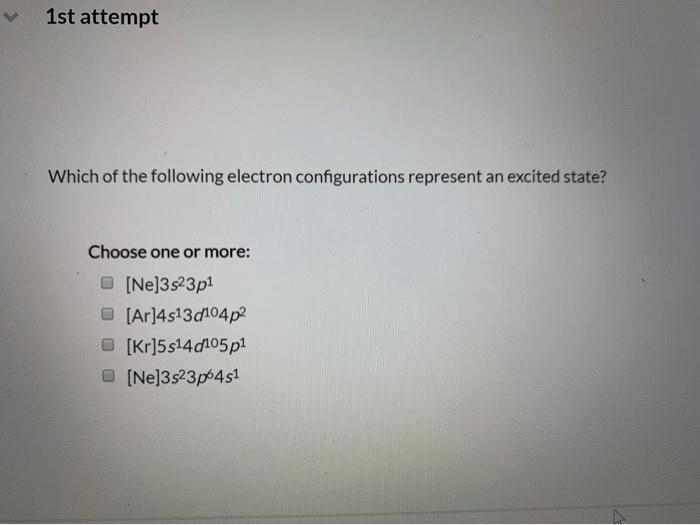
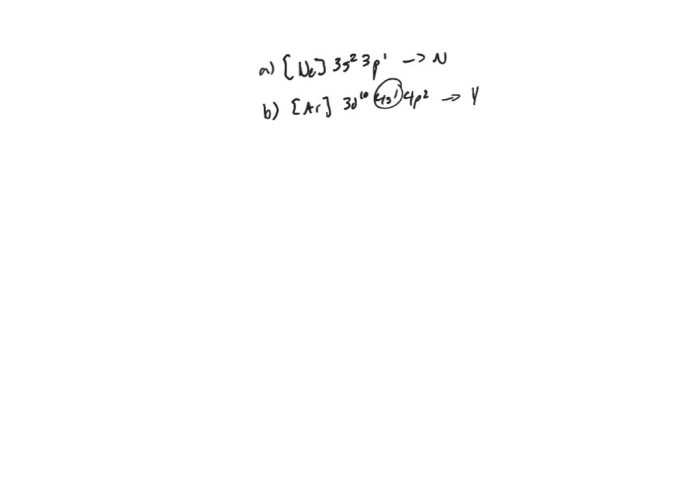Which of the following electron configurations represent an excited state? This question delves into the realm of electron behavior and the intricacies of energy levels within atoms. Join us as we unravel the characteristics, transitions, and applications of excited electron configurations.
Excited electron configurations arise when electrons occupy higher energy levels than their ground state. These configurations exhibit unique properties and play a crucial role in various scientific fields. Understanding the principles governing excited states is essential for comprehending atomic spectroscopy, chemical reactions, and advanced technologies.
Excited Electron Configurations
Electron excitation occurs when an electron gains energy, moving from its ground state to a higher energy level. This results in an excited electron configuration, which has a higher energy than the ground state configuration. Excited electron configurations are often unstable and decay back to the ground state, releasing energy in the form of light or heat.Excited
electron configurations can be identified by examining the electron configuration of an atom or ion. The ground state configuration is the lowest energy configuration, and excited states are configurations with higher energies. For example, the ground state configuration of helium is 1s 2, while an excited state configuration is 1s 12s 1.
Orbital Transitions and Energy Levels
Electron excitation involves the transition of an electron from one orbital to another. Orbitals are the regions around the nucleus where electrons are most likely to be found. Each orbital has a specific energy level, and electrons move to higher energy orbitals when they gain energy.The
energy difference between orbitals determines the wavelength of light that is emitted or absorbed when an electron transitions between them. For example, the transition of an electron from the 2s orbital to the 2p orbital in hydrogen emits a photon of light with a wavelength of 656 nm.
Excited State Stability and Decay
The stability of an excited electron configuration depends on several factors, including the energy difference between the excited state and the ground state, the number of electrons in the excited state, and the presence of other atoms or molecules.Excited states are typically unstable and decay back to the ground state within a short period of time.
The decay process can occur through a variety of mechanisms, including radiative decay, non-radiative decay, and autoionization.Radiative decay involves the emission of a photon of light as the electron transitions back to the ground state. Non-radiative decay involves the transfer of energy to another atom or molecule, without the emission of light.
Autoionization occurs when an electron is ejected from the atom or ion, leaving it in a positively charged state.
Spectroscopic Techniques for Excited State Analysis
Spectroscopic techniques are used to identify and analyze excited electron configurations. These techniques include absorption spectroscopy, emission spectroscopy, and photoelectron spectroscopy.Absorption spectroscopy measures the amount of light that is absorbed by a sample as a function of wavelength. Excited states can be identified by the absorption of light at specific wavelengths.Emission
spectroscopy measures the amount of light that is emitted by a sample as a function of wavelength. Excited states can be identified by the emission of light at specific wavelengths.Photoelectron spectroscopy measures the kinetic energy of electrons that are ejected from a sample when it is irradiated with light.
Excited states can be identified by the presence of electrons with specific kinetic energies.
Applications of Excited Electron Configurations, Which of the following electron configurations represent an excited state
Excited electron configurations have a wide range of applications in various fields, including chemistry, physics, and materials science.In chemistry, excited electron configurations are used to study the electronic structure of atoms and molecules. This information can be used to understand chemical bonding, reactivity, and spectroscopy.In
physics, excited electron configurations are used to study the properties of semiconductors and other materials. This information can be used to develop new electronic devices and technologies.In materials science, excited electron configurations are used to study the optical and electrical properties of materials.
This information can be used to develop new materials for use in solar cells, lasers, and other applications.
FAQs: Which Of The Following Electron Configurations Represent An Excited State
What factors influence the stability of excited electron configurations?
The stability of excited states depends on the energy difference between the excited and ground states, the availability of lower energy states for decay, and the presence of external factors such as temperature and magnetic fields.
How are excited electron configurations utilized in technologies?
Excited states play a vital role in lasers, fluorescent lighting, photovoltaics, and medical imaging techniques like MRI and PET scans.